Abstract
Objective:
Angioimmunoblastic T-cell lymphoma (AITL) is a common subtype of peripheral T-cell lymphoma (PTCL). AITL is an aggressive malignancy with a poor prognosis, and its clinical manifestations vary greatly among individuals. The current chemotherapy regimens based on anthracycline show limited efficacy, and there is no best rescue treatment for patients with relapsed and refractory (RR) AITL. In addition, the lack of optimal AITL models in vitro greatly limits the basic research on the mechanism of disease occurrence and progression, and also hinders the development of new drugs and preclinical trials. Our study aims to deeply analyze the tumor heterogeneity and clonal evolution of AITL, discovering key molecules of drug resistance and potential theraputic targets.
Methods:
We detected fresh lymph node samples from newly diagnosed and relapsed/ refractory AITL patients using single-cell RNA sequencing, combined with imaging mass cytometry (IMC) and whole exome sequencing. IMC was performed to analyze the spatial position relationship and protein expression characteristics of different subgroups in the tumor microenvironment of AITL. In addition, AITL patient-derived organoid model was established to study the regulatory role of YY1 and its inhibitors in relapsed and refractory AITL.
Results:
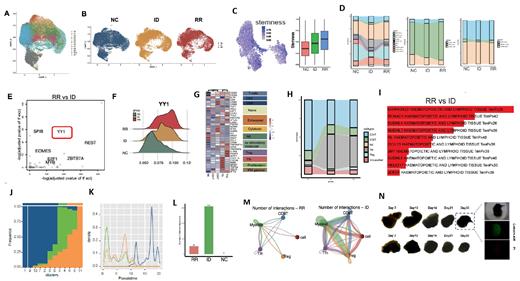
ScRNA-seq revealed the significant differences in the tumor microenvironment of newly diagnosed and RR-AITL patients (Fig A,B). B cells and myeloid subgroups may play important roles in the development of AITL (Fig C). Transcription factor YY1, highly expressed in follicular helper T cell (Tfh) of RR-AITL patients, promoted the proliferation and drug resistance of AITL cells (Fig E,F). The proportion of CD8+ T cells in the RR-AITL sample was reduced, while the proportion of Treg was increased, as well as the depletion of T cells (Fig G,H). Furthermore, the stemness of B cells in RR-AITL was enhanced and exhibits significant malignant characteristics (Fig C,I-K). We also found decreased interaction in RR-AITL samples (Fig L,M). Moreover, for the first time, we established AITL patient-derived organoid models that can be stablely cultured in vitro (Fig N). On this basis, we could further clarify the important roles of transcription factor YY1 in the drug resistance of AITL, evaluate the cytotoxic effect of YY1 inhibitor NP-001 on AITL tumor cells.
Conclusion:
In conclusion, our study revealed the differences between newly diagnosed and relapsed /refractory AITL in terms of immune microenvironment, single-cell transcriptomes, and signal pathway activation. YY1 may serve as an novel target for drug resistance for RR-AITL patients. These findings may provide a theoretical foundation for improving the clinical treatment of AITL.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal